Quote of the Day
I write a lot of programs and I can't claim to be typical but I can claim that I get a lot of them working for a large variety of things and I would find it harder if I had to spend all my time learning how to use somebody else's routines. It's much easier for me to learn a few basic concepts and then reuse code by text-editing the code that previously worked.
- Donald Knuth
Introduction

Figure 1: Tobacco is slightly radioactive. (Source)
I have been reading about the safety hazards associated with traveling to Mars. One of the hazards is radiation. Since I know very little about the biological hazards associated with radiation, I have some learning to do. One of the ways I learn about a subject is to work through problems from the various online and library references that are available. During my investigation, I came across four excellent articles on the subject of radiation exposure from smoking tobacco. So, if being unlikely to get a decent life insurance policy wasn't enough to keep you from giving up tobacco then hopefully this revelation will do the trick! This simple example illustrates the basic calculation process.
I will summarize the information here using a Fermi-type of analysis. As noted in the comments section, estimating the absorbed dose from the radiation activity level is never easy. My work here is very approximate, but does produce results in the same range as stated by the US National Institutes of Health. My overall objective is to build some tools to help me understand the effects that radiation in space and on Mars have on people.
Background
Reference Articles
My post was motivated by the following information I encountered on the web:
- New Times Article That Explains that Radiation is Always Present
- Good blog post on the topic with an excellent comment by David Gillies
- Environmental Protection Agency on Tobacco and Radiation
- Wikipedia article on the subject
Source of Radiation in Tobacco
The EPA addresses the source of the radiation from tobacco:
Naturally-occurring radioactive minerals accumulate on the sticky surfaces of tobacco leaves as the plant grows, and these minerals remain on the leaves throughout the manufacturing process. Additionally, the use of the phosphate fertilizer Apatite – which contains radium-226, lead-210, and polonium-210 – also increases the amount of radiation in tobacco plants.
The radium-226 that accumulates on the tobacco leaves predominantly emits alpha and gamma radiation. The lead-210 and polonium-210 particles lodge in the smoker's lungs, where they accumulate for decades (lead-210 has a half-life of 22.3 years). The tar from tobacco builds up on the bronchioles and traps even more of these particles. Over time, these particles can damage the lungs and lead to lung cancer.
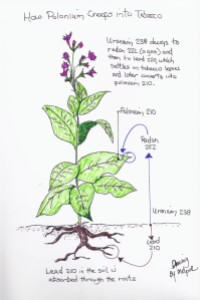
Figure 2 provides an excellent illustration of how polonium-210 (210Po), uranium-238 (238U), and lead-210 (210Pb) get into tobacco (Source: Mel Porter).
Appendix A goes into detail on how 210Po actually gets into the leaves because of 222Rn.
I found a number of quite different values quoted for the radiation level of tobacco leaves. I decided to choose the value that reflected the average radioactivity levels for US tobacco. US tobacco is more radioactive than others, possibly because of our use of slightly more radioactive fertilizers.
Radiation Exposure Definitions
Types of Radiation Doses
- Absorbed dose
- Energy absorbed by a kg of a substance. The absorbed dose is represented symbolically by DT,R, with T representing the specific tissue (e.g. brain) and R representing the specific type of radiation (e.g. x-ray). Absorbed dose is measured in units of Gray (Gy). By definition, 1 Gy = 1 joule/kg.
- Equivalent dose
- Equivalent dose is the absorbed dose weighted by the effect of the different types of radiation. The equivalent dose is represented symbolically by HT and computed by the formula
, where wR represents the weighting for radiation effects relative to x-rays (wX-Rays=1). Equivalent dose is measured in units of Sieverts (Sv).
- Effective dose
- Effective dose is the equivalent dose weighted by the radiation sensitivities of the different tissues. The effective dose is represented symbolically by E and computed by the formula
, where wT represents the weighting for tissue radiation sensitivity. The tissue radiation sensitivity is normalized so that weights for all tissues sum to 1. Effective dose is measured in units of Sieverts (Sv).
Radiation Units
- Sievert
- The Wikipedia defines the Sievert (symbol: Sv) as the SI derived unit of equivalent radiation dose. The Sievert represents a measure of the biological effect, and should not be used to express the unmodified absorbed dose of radiation energy, which is a physical quantity measured in Grays.
- Gray
- The Gray (symbol: Gy) is the SI derived unit of absorbed dose. Such energies are typically associated with ionizing radiation such as X-rays or gamma particles or with other nuclear particles. It is defined as the absorption of one joule of such energy by one kilogram of matter.
Analysis
My calculations use the same approach as David Gillies in his forum posting. However, my inputs ended up being different and I obtained a different result.
Discussion of Steady State Radiation Level
Over time, the radiation level emitted from cigarette smoking approaches a steady-state level. The steady state level is reached when the 210Po that decays each day is exactly cancelled by the amount of 210Po that is being inhaled every day. My analysis assumes that the smoker has reached steady state.
Definition of Units
Figure 3 shows the various units that I defined for this problem solution.
Weighting of Effects By Radiation Type
Figure 4 shows the biological weighting factors for different kinds of radation. 210Po emits alpha particles, which have a weighting factor of 20 relative to x-rays.
Polonium Characteristics
Figure 5 shows the relevant facts on 210Po.
Radiation Model
Figure 6 shows my calculations for the effective radiation dose that a 1.5 pack a day smoker endures.
Conclusion
I was looking for a simple example of computing the effects of radiation on a human. This example produces a result that is consistent with the data in the Wikipedia.
Appendix A: Source of Polonium
210Po is generated as a decay product from 222Rn. Here is the decay chain for 222Rn, which has 210Po as an intermediate product (Source). I highlighted the isotopes mentioned above.
- 222Rn, 3.8 days, alpha decaying to...
- 218Po, 3.10 minutes, alpha decaying to...
- 214Pb, 26.8 minutes, beta decaying to...
- 214Bi, 19.9 minutes, beta decaying to...
- 214Po, 0.1643 ms, alpha decaying to...
- 210Pb, which has a much longer half-life of 22.3 years, beta decaying to...
- 210Bi, 5.013 days, beta decaying to...
- 210Po, 138.376 days, alpha decaying to...
- 206Pb, stable.





Pingback: Radiation Exposure on a Trip to Mars | Math Encounters Blog
Your calculations, although formally correct, do not take into account the dose conversion coefficients, which depend on "parameters such as the inhalation speed through the mouth, the real fraction of radionuclide transferred from cigarette to mainstream smoke, the lung absorption behavior of the radioisotopes inhaled with mainstream smoke, etc..." (from Taroni et al. Health Physics, 107 (2014) p 109)
If one consider these factos, the Sieverts obtained are about 500 smaller than the value you get by assuming that all the polonium is absorbed in the lungs.
By the way, it's never straightforward to transform activity into an absorbed dose.
I understand that these are complex calculations with many variables. I was just interested in where a number in the Wikipedia came from – apparently they used a similar analysis. I have been reading some documents from Oak Ridge on the subject of radiation exposure from common material and that is what motivated me to attempt some simple calculations. My main interest has been to develop some familiarity with the calculations as they relate to space travel.
As far as the effects of smoking in general, I have read some work that indicated that even the resistance provided by a cigarette's filter can cause people to inhale deeper and drive the toxins further into the lungs. So even the cigarette's construction makes a difference. These are very complex things to model.
Thanks for your comment.
mathscinotes
Also good Reading
p237
https://www-pub.iaea.org/MTCD/Publications/PDF/D484_web.pdf
Ref.:
There are several publications which deal with 210Pb and 20Po in tobacco and the transfer to humans (see Table V.1). Activity concentrations are in the range of 2.8–37 mBq/g. There are few data on activity concentrations in pipe tobacco, cigar tobacco and snuff. A cigarette contains about 1 g of tobacco.